PHY 055-01
Course Syllabus Spring 2013
MWF 8:00 - 9:15 am 120 Olson Hall Dr. Joseph F. Alward
Office Telephone: (209) 946-3129
Email Address: JFAlward@aol.com
Textbooks:
"Fundamentals of Physics," by Halliday, Resnick, Walker, 9th Edition.
"Physics for Engineering and Science," by Michael Browne. Publisher: Schaum's Outline Series.
There is a great deal more information on the pages of the Resnick-Halliday chapters than you are required to know. The Schaum's text better represents the content you need to understand, while the Resnick-Halliday text will provide deeper insight into the concepts than does the Schaum's text.
Study only the content in the Halliday-Resnick chapters that relate to lecture material. Attempt to work only problems without an asterisk, and only those problems that are odd-numbered (for which the answers are provided at the end of the textbook).
A recommended on-line resource is HyperPhysics, an educational website covering physics topics, hosted by Georgia State University.
The first part of this course covers heat and thermodynamics. To supplement the heat and thermodynamics material presented in lecture and the textbooks, students may click on any of the topics in the HyperPhysics "tree" below. Not all of the topics in the tree are covered in this course. Click once anywhere on the tree below, then click again on the specific topic when you arrive at the same tree on the HyperPhysics web page.

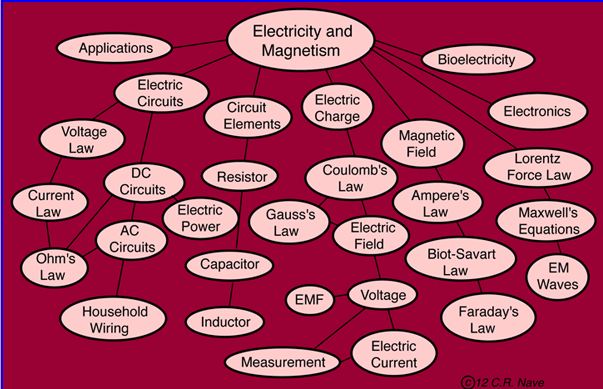
The remainder of this course deals with electromagnetism. To supplement the electromagnetism material presented in lecture and the textbooks, students may click on any of the topics in the HyperPhysics "tree" below. Not all of the topics in the tree are covered in this course. Click once anywhere below, then click again on the specific topic when you arrive at the same tree on the HyperPhysics web page. Not all of the topics displayed in the tree below are covered in this course.
Course Schedule:
Lectures will be given on the days listed in the table below. Holidays are indicated, as well as the dates of the four examinations and final exam.
Monday |
Wednesday |
Friday |
Jan 7 |
Jan 9 |
Jan 11 |
| Jan 14 |
Jan 16 |
Jan 18 |
| Jan 21 Holiday |
Jan 23 |
Jan 25 |
| Jan 28 Exam 1 (See details below) |
Jan 30 |
Feb 1 |
| Feb 4 |
Feb 6 |
Feb 8 |
| Feb 11 |
Feb 13 |
Feb 15 |
| Feb 18 Holiday |
Feb 20 |
Feb 22 Exam 2 (see details below) |
| Feb 25 |
Feb 27 |
Mar 1 |
| Mar 4 Holiday |
Mar 6 Holiday |
Mar 8 Holiday |
| Mar 11 |
Mar 13 |
Mar 15 |
| Mar 18 |
Mar 20
|
Mar 22 |
| Mar 25 Exam 3 (See details below) |
Mar 27 |
Mar 29 Holiday |
| Apr 1 |
Apr 3 |
Apr 5 |
| Apr 8 |
Apr 10 |
Apr 12 |
| Apr 15 |
Apr 17 |
Apr 19 |
| Apr 22 Exam 4 (See details below) |
Apr 24 Classes End |
|
| |
May 1 Final Exam 8-11 am |
|
Final Examination
According to the schedule shown on the website at the address below, the final exam for Physics 55 will be given on Wednesday, May 1, 2013, from 8:00 am to 11:00 am. Students should confirm this information by clicking on the "Final Exam Schedule" link below to make sure there have been no changes.
Final Exam Schedule
Course grades will be online beginning Tuesday, May 7, 2013.
The list below of material that will be covered on the various examinations is not exhaustive. Other material, not listed below, which may have been discussed in lecture, may also appear on the examinations.
Examinations: (500 points) There are four 75-minute, 100-point, examinations, and a 100-point final exam. The exams covers the material described below for each exam.
Students may use one side of a sheet of 8 ½ x 11 inch paper containing any type of information to assist them during examinations.
The final examination is worth 100 points, and covers all of the material in the course. Students may use four one-sided sheets of notes to assist them during the final examination, or two sheets with notes on each side of each sheet.
Students must not have any sort of electronic communication device within reach at any time during the examinations. Sharing of calculators is also strictly forbidden, as is any other method of sharing information. Such activity will be regarded as a violation of the College's Honor Code Policy:
|
Exam 1: Monday,
January 28, 2013
Exam 1 Fall 2012
Recommended Reading in the Schaum's text
:
Chapter 17: Note: Problem 17.9 is worked differently than the way we work similar problems in lecture. In lecture, the sum of all of the Q's equals zero, so rather than equate the heats gained to the heats lost, which requires that some of the temperatures be subtracted in the reverse order from the proper final minus initial order, in order to create positive values, in lecture we just add all of the Q's, and always calculate the changes in temperatures by subtracting the initial from the final. In this way, some of the Q's will be positive and some naturally, automatically.
Ignore Equation 17.17.
Errors in the Schaum's Chapter 17:
Problem 17.18. Equation should be ΔL, not ΔV. The correct numbers should be 2.0005 cm and 1.9995 cm.
Problem 17.20. It should be, "Heat lost by person = Heat gained by water."
Problem 17.25. The correct heat flow through the crust is 54 mW (milli-watts) per square meter.
Chapter 18: Section 18.1 only. Do Supplementary Problems 9, 11, and 12. The answer given for Problem 12 should be 0.00124 per cubic centimeter.
Chapter 19: Skip this chapter
Chapter 20: Skip page 226 and Problems 15, 17,18, 19, 25, 26 and 27. Typo in Problem 20.15: "magnetic" should be "electric."
Recommended reading in the Halliday-Resnick textbook:
Chapter 18: Temperature scales, thermal expansion, internal energy and heat, specific heat capacity, phase changes, work done during volume changes, First Law of Thermodynamics, conduction, convection, radiation.
Chapter 19:
Moles, ideal gas law, work done by an expanding ideal gas, kinetic energy of ideal gas molecules.
Chapter 20: Skip
Chapter 21: Electric charge, conductors and insulators, Coulomb's Law.
Chapter 22: Electric fields due to: point charges, several point charges, and continuous charge distributions.
Exam 2: Friday, February 22, 2013
Exam 2 Fall 2012
Recommended reading in Schaum's "Physics for Engineering and Science":
Chapter 21. Skip Problem 21.5, 21.16, 21.20, and 21.23. Problem 21.21 contains two errors. First, the is an "r" in in the denominator of the integrand of the first equation that does not belong there, and the 1/2 in the resulting expression also doesn't belong there.
Chapter 22. Skip content dealing with spherical coordinates (Equation 22.13); skip Problems 22.3, 22.4, 22.9, 22.19.
Chapter 23. Skip Problems 2, 3, 13, 23, 20, and 22. There is an error in Problem 21.24: there is an a-squared missing in the denominator of the answer.
Related material in the Halliday-Resnick textbook:
Chapter 23: Flux of an electric field, Gauss' Law.
Chapter 24:
Electric potential energy of: a single point charge, a set of point charges. Electric potential: at a point, due to one or more point charges or a continuous distribution of charge. Electric potential functions. Determining the electric field value from the electric potential function. Equipotential lines, curves and surfaces. Electric field and potential of a charged conductor.
Chapter 25: Capacitance, parallel plate capacitors, cylindrical capacitors, spherical capacitors, capacitors in series, in parallel, potential energy stored in a capacitor.
Exam 3: Monday, March 25
Exam 3 Fall 2012
Recommended reading in the Schaum's text:
Chapter 24 (skip Problem 5,12, 16, 20, 22). Problem 14 incorrectly uses 300 seconds instead of 360 seconds.
Chapter 25 (skip Problems 4, 8, 13, 17, 18).
Chapter 26 (skip Section 26.4 and skip the equations in the Summary of Section 26.5 that deal with magnetic moment and torque on a loop). Skip Supplementary Problems 19, 20.
Recommended reading in the Halliday-Resnick text:
Chapter 26: Electric current, resistance, Ohm's Law, current density, resistivity, conductivity, drift velocity, battery output power, power dissipated in a resistor.
Chapter 27:
EMF, loop rule (Kirchoff's law), internal resistance, resistors in series and parallel, RC circuits.
Chapter 28: Magnetic fields, magnetic force on moving charges, magnetic force on current-carrying wires. There is an error in Problem 28: the equation should not include a "ln" in front of (-t/tau).
Exam 4: Monday, April 22
ERROR IN GRADING EXAM 4:
The correct answer to Problem 14 should be (b), not (a). Whatever score was place on your exam, it's been increased by 5.
Exam 4 Fall 2012
Note: there is an error in Problem 14: the correct answer is "east," not "north." Also, in Problem 11, all of the answers on the list are missing a "4 pi" in the denominators. In Problem 7, the 4 pi in the denominator of Answer (d) should be 2 pi.
Recommended reading in the Schaum's text:
Chapter 27: Skip Skip problems 9, 15, and 19. Problem 12 is related to Problem 21; looking at Problem 21 may give you clues as to how to approach Problem 12. Ignore the equations in the Summary dealing with the "plane sheet" and "on the axis of circular loop."
Skip Problems 27.13, 23.17, 27.18.
Chapter 28: Skip Sections 28.5.. Skip Problems 28.5, 28.6, 28.8, 28.9, 28.10, 28.11, 28.17, 28.18, 28.19, 28.21, 28.23, and 28.27.
Chapter 29: Skip
Recommended reading in the Halliday-Resnick text:
Chapter 29: Law of Biot-Savart, magnetic field due: a long straight wire, a circular loop of current-carrying wire. Magnetic force between parallel currents, Ampere's Law.
Chapter 30: Magnetic flux, Faraday's Law of Induction, Lenz's Law, inductors, inductance, self-induction, RL circuits, potential energy of an inductor carrying current.
Chapter 31: LC oscillations, alternating currents and alternating current power supplies, RMS current, RMS voltage, power.
Final Exam: Wednesday, May 1, 8:00 am - 11:00 am
Fall 2012 Final Exam
The final exam covers all of the course material.
Violations of the Honor Code
The examinations are "curved," which means that points will be added to everyone's exam depending on the class average, in order to raise the class average; some students might earn more than the 100 points "possible" on some exams. The higher the class average, the fewer will be the number of points added in the curve, and the lower will be everyone's score. It is therefore in each person's interest that the class average relative to their own score be low. Students who are perceived as taking an unfair advantage during examinations, thereby raising the class average, are usually reported to the professor by other students. It is therefore important that all students make certain not to be seen by other students using a cell phone or any other communication device during examinations, or looking at another student's answer sheet. Several times in the past, following several complaints by observant students, investigations of students' use of cell phones during exams were conducted, with the result that the offending students were reported to the Office of Judicial Affairs. Almost every semester in every class students report violations to the professor and, following Judicial Affairs Hearings,the offending student is given a zero on the exam, or worse. Do not be seen using a cell phone during exams, or be seen talking to other students, because someone is certain to report you.
Other examples of violations of the honor code include, but are not limited to, verbal communications with other students during exams, and using more than the allowed amount of notes. On each of the four examinations, students may use one side of one sheet of paper filled with any information; using more than one side of one sheet is a violation of the honor code. On the final exam, students may use four sides of four sheets of paper, or two sides of two sheets, or other combinations of sides that do not total more than four sides.
The College of the Pacific Honor Code Policy
Approved by COP Council: November, 2009
The College of the Pacific holds all of its students to a strict standard of academic integrity. In the case of a suspected violation of the University academic honor code, the faculty member and the chair of the department will evaluate the alleged infraction and report it immediately to both the chair of the department, the College Academic Affairs office, and the Office of Judicial Affairs, which will begin a formal investigation.
If the Office of Judicial Affairs determines that the student was is responsible for the honor code violation, the standard penalty in the College is failure of the assignment and/or the course. In such cases, the student will be prevented from dropping or withdrawing from the course, even if the deadline to do so has not expired. Further disciplinary action may also be taken by the Office of Judicial Affairs |
|
Grading
Laboratory: (120 points)
Students meet 12 times to work in teams to complete laboratory exercises related to the concepts discussed in the lectures. There are 10 points possible for each lab meeting. Bring a calculator to every session. No laboratory notebook or text is required; no advance work is necessary and all work completed during the lab period is turned in at the end of the period. There are no make-up labs.
Grading
Laboratory: (120 points)
Students meet 12 times to work in teams to complete laboratory exercises related to the concepts discussed in the lectures. There are 10 points possible for each lab meeting. Bring a calculator to every session. No laboratory notebook or text is required; no advance work is necessary and all work completed during the lab period is turned in at the end of the period. There are no make-up labs.
Replacing the Two Lower Exam Scores
The two lower of the four exam scores will be replaced by the final examination score, provided the final exam score is higher than the lower scores; if the final exam score is not higher than the lower scores, no replacement will be made. For example, if a student received 40 and 50 on the first two exams, but got a 90 on the final exam, those two exam scores will be erased and replaced by 90. Thus, a student who had two of the four exam scores in the "failing" range can still earn an "A" in the course.
Any examination score that is voided as the result of a proved violation of the Student Honor Code will not be replaced by the final examination score.
Curving the Exams
The exams are curved, depending on the class average. Some students may receive more than 100 points on a 100-point exam because of their additional points that might have been added to everyone's score in the curving process.
Some test-taking advice
Quickly scan the exam problems, taking note of those which you think are the harder problems. Save those for last. It's very important that you not spend too much time on any one problem as this might prevent you from discovering two or three simpler problems you have not yet worked, but which can quickly be answered. Look for the easy problems, and work those first. The worst thing that can happen to test-takers is to run into a difficult problem early in the exam period, waste several minutes on it, still not get the answer, and not only lose all that time, but lose considerable confidence as well, and then rush to make up lost time and in their haste make mistakes.
The final examination score will replace the lower two of the four previous exam scores, provided the final exam score is higher than those scores. There are no make-up, late, or early exams; if a student misses an exam, that exam will count as the student's lowest exam score.
There are 500 points possible for the five exams; some students, because of curving, may actually receive more than 500 points for the five exams.
There are 120 points possible for the lab, for a total of 620 points. Your course grade will be based on following percentage scale:
92-100: A
90-92: A-
88-90: B+
82-88: B
80-82: B-
78-80: C+
72-78: C
70-72: C-
68-70: D+
60-68: D
0-60: F
In the event your course percentage falls exactly on the dividing line, such as 92.00, for example, you will be given the higher grade (in this example, A). Course percentages are not rounded, so if your course percentage, for example, is 89.9999%, your course grade will be a B+. If it is exactly 90%, your course grade is an A-.
A student whose average on the five exams is, say, 77 (a "C"), but who collects the typical number of points in the laboratory (about 115 out of 120), would have a course total of 500 points out of 620, or 80.65% (a B-). This example illustrates how important the lab score is in determining the student's final grade. Attend lab, finish it (everyone does), and you will probably earn about 115-120 points, which usually makes a huge difference in your course grade.
Frequently Asked Questions Regarding Grading
"What grade do I need on the final to get a B (for example)?"
A hypothetical student got 114 points out 120 points in the lab, and the following scores on four exams: 48 67 75 86. She or he needs 82% of 620 points, or 0.82 x 620 = 508.40. Assume that the final exam score will be higher than the "48" and "67" received on two of the exams. Let x = final exam score. The five exam scores, plus the lab score, must be at least 509:
x + x + 75 + 86 + x + 114 = 509. Solving for x, we get x = 78. If the student gets 78 or higher on the final exam, he or she will be guaranteed at least a B in the course.
|